
ClinXTrials
A Vienna based alliance to accelerate clinical development

Clinical Development
Analytics
Logistics
In the competitive world of biotechnology, early-stage companies face immense challenges in translating innovation into successful clinical programs. That’s where 𝗖𝗹𝗶𝗻𝗫𝗧𝗿𝗶𝗮𝗹𝘀 steps in - a powerhouse alliance uniting deep expertise in 𝘤𝘭𝘪𝘯𝘪𝘤𝘢𝘭 𝘥𝘦𝘷𝘦𝘭𝘰𝘱𝘮𝘦𝘯𝘵, 𝘣𝘪𝘰𝘢𝘯𝘢𝘭𝘺𝘵𝘪𝘤𝘴, and 𝘵𝘳𝘪𝘢𝘭 𝘭𝘰𝘨𝘪𝘴𝘵𝘪𝘤𝘴 to provide an integrated, end-to-end solution.
Together, under the 𝗖𝗹𝗶𝗻𝗫𝗧𝗿𝗶𝗮𝗹𝘀 banner, we offer a seamless pathway from early strategy to study execution - accelerating timelines, reducing complexity, and helping our clients bring life-changing therapies to patients more efficiently. Based in the heart of Europe, we truly believe that this alliance will add great value to Vienna as site for clinical development.
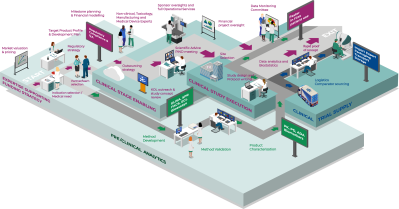
3 Companies - One Vision
Gouya Insights are strategic clinical development experts working with biotech, academic start-ups, pharmaceutical and device companies. Gouya Insights will translate your innovation into a clinical roadmap and design the most efficient clinical development plan. Our experienced and multidisciplinary team provides innovative strategies to meet your clinical milestones and product goals.
VelaLabs is GLP certified and GCLP compliant laboratory for the bioanalytic testing of pre/clinical samples, from early stage up to Phase III studies. In addition, VelaLabs' GMP certification allows product characterisation assays of biomolecules, as well as QP release of clinical and market material. Tailor-made pharmacokinetics, pharmacodynamics and immunogenicity (anti-drug antibody) assays can be developed and validated according to the relevant EMA and FDA guidelines and white papers. Our track record spans more than 60 studies, covering a broad spectrum of indications, products, sponsors, and clinical phases.
ABF Pharmaceutical Services is a GMP- and GDP-certified service provider, specialising in comprehensive clinical trial supply solutions. With a strong focus on small-batch projects, we offer tailored services for the primary and secondary packaging, labeling, storage, and distribution of investigational medicinal products (IMPs) and clinical trial materials. In addition to our IMP services, ABF provides specialised capabilities in the processing, storage, and logistics of Peripheral Blood Mononuclear Cells (PBMCs).